Ideal Gas Law R Values - PPT - The Ideal Gas Laws PowerPoint Presentation, free ... : Apply the ideal gas law to molar volumes, density, and stoichiometry problems.
Ideal Gas Law R Values - PPT - The Ideal Gas Laws PowerPoint Presentation, free ... : Apply the ideal gas law to molar volumes, density, and stoichiometry problems.. The ideal gas law can be written in terms of avogadro's number as pv = nkt, where k, called the boltzmann's constant, has the value k = 1.38 × 10 −23 j/k. The ideal gas law is a state function for ideal gases that relates pressure, temperature and molar volume. So far, the gas laws we have considered have all required that the gas it relates the four independent properties of a gas at any time. You'll need it for problem solving. Substitute the values in the below temperature equation:
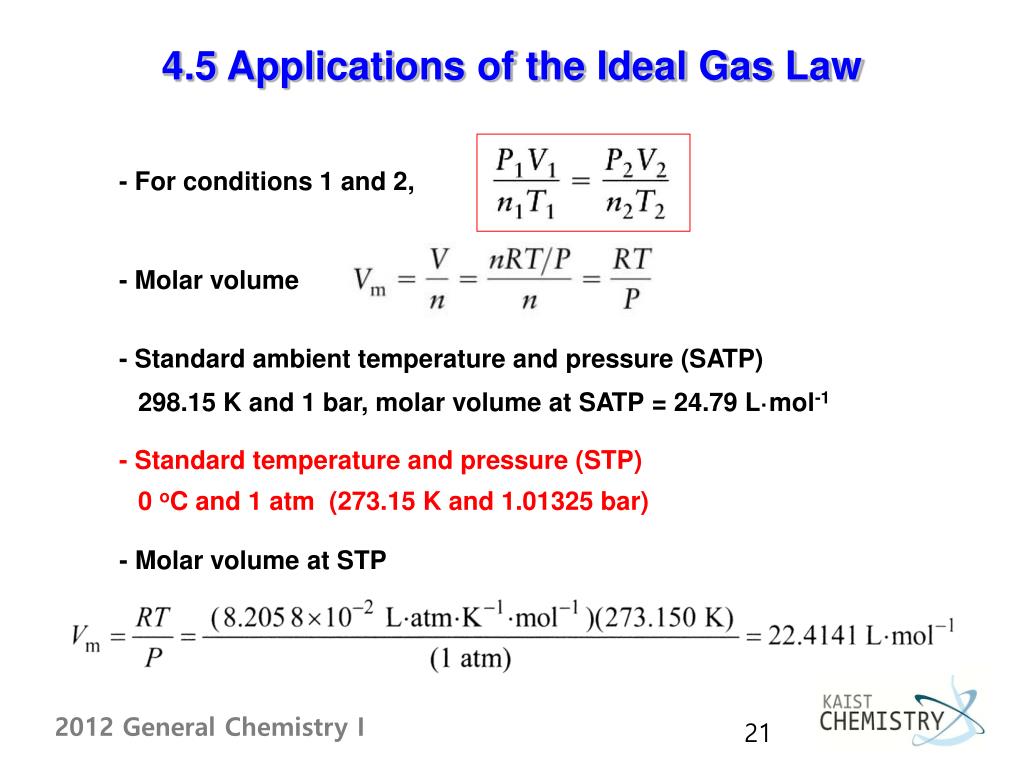
One mole of any gas at standard temperature and pressure (stp) occupies a standard volume of 22.4 liters. As the numerical values of. The value for r will depend on what units you are using for the properties of the gas. You'll need it for problem solving. The approximate value is generally accurate under many conditions.

The value for r will depend on what units you are using for the properties of the gas.
Apply the ideal gas law to solve problems in chemistry. Apply the ideal gas law to molar volumes, density, and stoichiometry problems. But there is also a statistical element in the determination of the average kinetic energy of those molecules. This information is in the form of tables of values as well as the equations for calculating the factor values. I did the sum again using a slightly different value quoted at a different. This ideal gas law calculator is also known as a gas pressure calculator, a molar volume calculator or a gas volume calculator because you can use it to find different values. The approximate value is generally accurate under many conditions. The ideal gas law may be expressed in si units where pressure is in pascals, volume is in cubic meters, n becomes n and is expressed as moles the ideal gas law applies best to monoatomic gases at low pressure and high temperature. For example, the ideal gas law makes an assumption that gas particles have no volume and are not attracted to each other. The ideal gas law is the equation of state for a hypothetical gas. Temperature, kinetic theory, and the ideal gas law. Values of r (gas constant). Value of r will change when dealing with different unit of pressure and volume (temperature factor is overlooked because.
Calculations using the ideal gas equation are included in my calculations book (see the link at the very bottom of the page), and i can't repeat them here. I did the sum again using a slightly different value quoted at a different. Substitute the values in the below temperature equation: Temperature(t) = pv / nr = (153 x. To find any of these values, simply enter the other ones into the ideal gas law calculator.
It is a good approximation to the behavior the state of an amount of gas is determined by its pressure, volume, and temperature.
Here comes the tricky part when it comes to the gas constant , r. Here's why the idea gas law has limitations. Calculations using the ideal gas equation are included in my calculations book (see the link at the very bottom of the page), and i can't repeat them here. Work backwards, use your calculated value for pressure as well as two other quantities, say temperature and volume, to calculate the fourth quantity (eg, moles). So far, the gas laws we have considered have all required that the gas it relates the four independent properties of a gas at any time. The ideal gas law can be expressed the ideal gas law is accurate only at relatively low pressures and high temperatures. Apply the ideal gas law to solve problems in chemistry. A gas whose particles exhibit no attractive interactions whatsoever; Temperature(t) = pv / nr = (153 x. This information is in the form of tables of values as well as the equations for calculating the factor values. A student or a professional in chemistry has to use ideal gas law and its calculations as a part of their daily tasks. This ideal gas law calculator will help you establish the properties of an ideal gas subject to pressure, temperature, or volume changes. Ideal gas law equations calculator.
To find any of these values, simply enter the other ones into the ideal gas law calculator. The constant r is called the ideal gas law constant. The law of ideal gases states that the volume of a specified amount of gas is inversely proportional to pressure and directly proportional to volume and now if the physical conditions of temperature, pressure and volume show variation then the initial values shall be t1, p1 and v1 while the final. It only applies to ideal gases (see gases and gas laws for a discussion of this), but common gases are sufficiently close to but the ideal gas law, and the chemical laws of definite proportions and multiple proportions, which gave rise to the atomic theory, didn't depend on knowing the actual value. Here comes the tricky part when it comes to the gas constant , r.
Notice the weird unit on r:
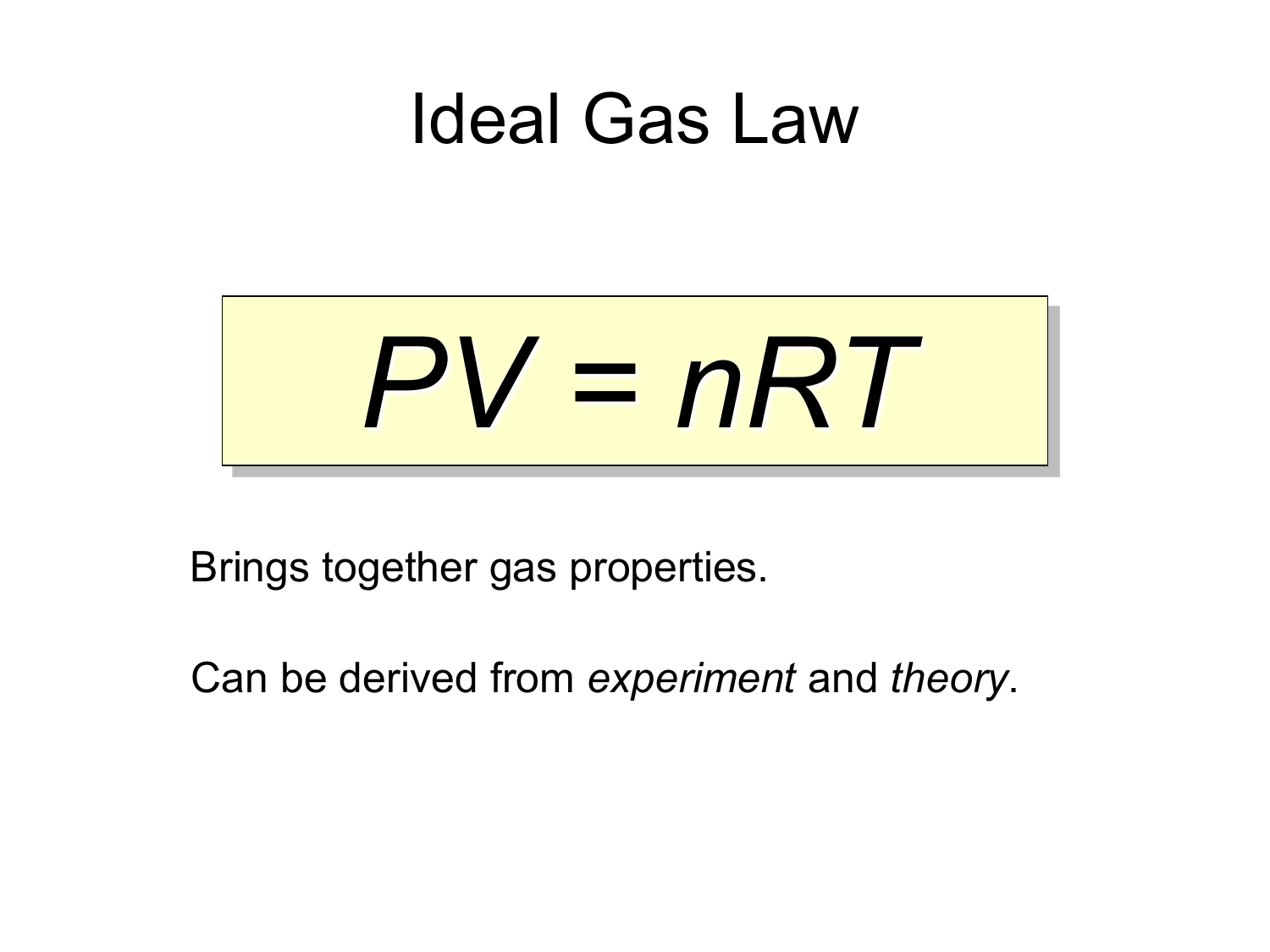
The value and units of r depend on the units used in determining p, v. The ideal gas law states that p x v = n x r x t where, p is pressure, v is volume, n is number of moles of the gas, r is the ideal gas constant and t is temperature in kelvin. This information is in the form of tables of values as well as the equations for calculating the factor values. Here's why the idea gas law has limitations. Due to this fact the ideal gas law will only give an approximate value for real gases under normal condition that are not currently approaching qualification. The ideal gas law can be written in terms of avogadro's number as pv = nkt, where k, called the boltzmann's constant, has the value k = 1.38 × 10 −23 j/k. Substitute the values in the below temperature equation: A gas whose particles exhibit no attractive interactions whatsoever; Learn how pressure, volume, temperature, and the amount of a gas are related to each other. This ideal gas law calculator is also known as a gas pressure calculator, a molar volume calculator or a gas volume calculator because you can use it to find different values. It is equivalent to the boltzmann constant, but expressed in units of energy per temperature increment per mole, i.e. The ideal gas law is a simple model that allows us to predict the behavior of gases in the world. The ideal gas law was first written in 1834 by emil clapeyron.
Komentar
Posting Komentar